Research Overview
The microenvironment of human tumors is unlike that of any normal tissue, characterized by extreme heterogeneities in nutrient supply, pH, and oxygenation. These features develop as a consequence of alterations in the metabolic and proliferative status of tumor cells together with a highly irregular vascular supply. Our group is investigating the tumor microenvironment with a primary interest in understanding the cellular and molecular responses to deficiencies in oxygenation (hypoxia) and their influence on the biological behavior of tumors.
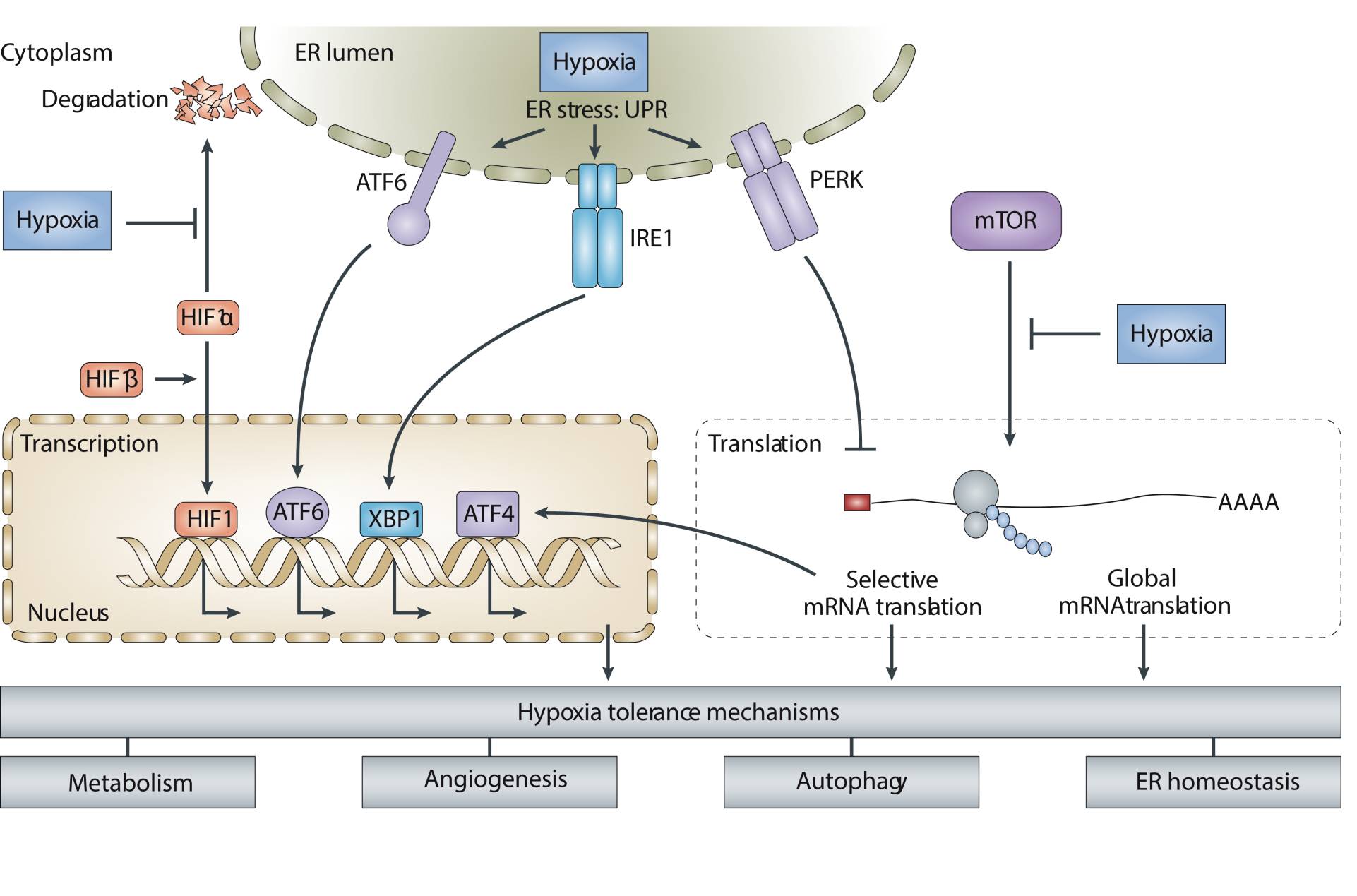
Both laboratory and clinical evidence strongly associate hypoxia with tumor development, tumor growth, metastasis and poor response to therapy. Our group has identified two important pathways that independently influence gene expression and processes of importance for hypoxic tumor cell behavior. The first of these occurs through regulation of an important integrator of metabolic signals, the mammalian target of rapamycin (mTOR) kinase and its downstream effectors that orchestrate the initiation of protein synthesis, autophagy and apoptosis sensitivity. The second is through activation of the unfolded protein response (UPR), a program of transcriptional and translational changes that occurs as a consequence of endoplasmic reticulum (ER) stress. The UPR controls multiple downstream processes including protein production, maturation and degradation, cell metabolism and cell death. Using genetic and biochemical approaches, our lab has demonstrated the importance of these pathways in regulating gene expression, hypoxia tolerance, tumor growth and response to treatment.
Our group also has interests in DNA repair, ubiquitin signaling, and is involved in translational research incorporating molecular-targeted agents. We try to work closely with clinical colleagues to establish high content clinical trials in radiation oncology and to translate our basic research findings into the clinic.
Ongoing Projects
- Defining the molecular basis of oxygen sensing in the pathways that regulate mTOR and UPR signaling during hypoxia. We are currently studying the contribution of oxygen to protein folding and maturation in the endoplasmic reticulum.
- Determining changes in gene expression downstream of hypoxic signaling pathways. These studies utilize high-throughput genomic methods to characterize changes in transcription, translation and protein production. Our group believes that hypoxia-regulated proteins will be useful for both diagnostic and therapeutic purpose.
- Understanding how hypoxic response pathways influence the biology of tumor cells in ways that affect patient prognosis. The signaling pathways regulated by oxygen control multiple cellular processes that are important for tumor cell growth, response to stress, and survival. These include regulation of metastasis, DNA repair, autophagy, and metabolism.
- Assessing the potential of targeting hypoxic responses for improved treatment of cancer. This work involves the creation of tumor models that allow genetic manipulation of various components of hypoxic response pathways in established tumors in mice. The importance of different hypoxic response pathways is evaluated by monitoring changes in tumor metabolism, the tumor microenvironment, tumor growth, and response to treatment.
- Discovery of vulnerabilities in cancer that are manifested only within the unique microenvironment of tumors. Our group believes that cancer-associated genetic alterations influence tumor hypoxia and metabolism in ways that can be exploited for therapy and we are using high-throughput genomic tools such as RNA interference screens to identify novel molecular targets.